Volunteers Needed for PET Imaging Study!
M
Mark Green
Primary Investigator
Overview
Why is this study being done?
Researchers at Indiana University School of Medicine are studying the use of a new imaging agent called Cu-ETS (Copper-ETS) in positron-emission tomography (PET) to detect blood flow in normal tissues. An imaging agent is a tracer that is a special dye containing
radioactive tracers that are either swallowed, inhaled, or injected intovein in your arm depending on what part of the body is being
examined. When detected by a PET scanner, the tracers help your doctor
to see how well your organs and tissues are working.
Copper-ETS is not approved by the Food and Drug Administration (FDA),
however it has been approved by the Radioactive Drug Research Committee
(RDRC) for use in this research.
We hope to learn more about whether Copper-ETS PET will provide useful information about the blood flow in people with normal tissues, as well as about patterns of blood flow in people who have tissue disturbed by medical conditions, such as cancer and heart disease.
Who can take part in this study?
Adults who are 20-80 years of age and are able to complete a PET scan at Goodman Hall on IUPUI in Indianapolis, IN (if female, must not currently be pregnant).
Description
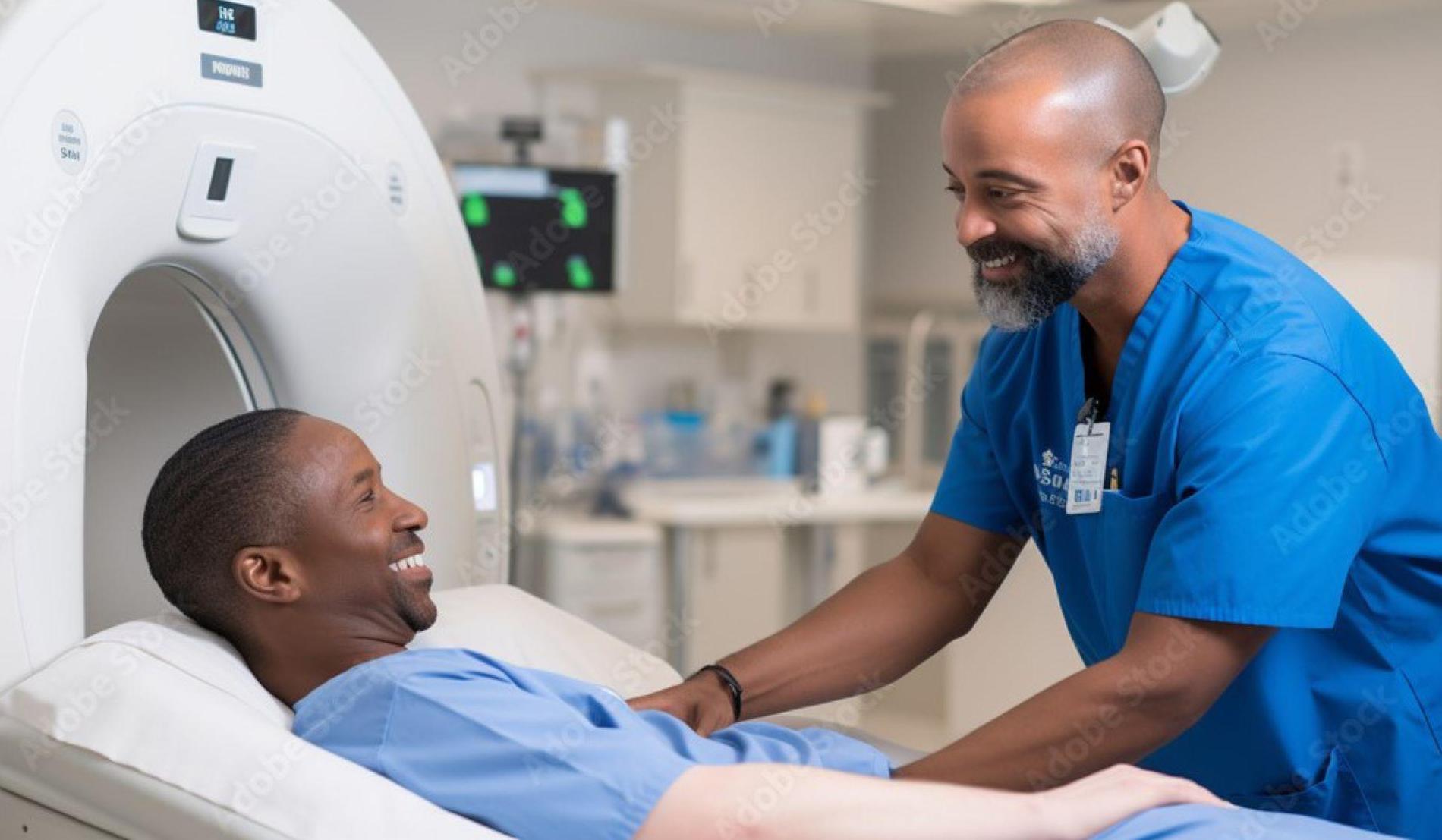
What will happen during the study?
You will be scheduled to receive a PET/CT scan, at the PET facility in Goodman Hall on IUPUI campus in Indianapolis, IN.
**If you are female and can have children, a urine pregnancy test will be done prior to the PET scan to make sure you are not pregnant.
The PET/CT scan study visit will last approx. 2.5 hours and involves the following steps:
a. An intravenous tube, or catheter, will be placed in one of your arm veins to allow for injection of the radioactive substances. A second intravenous tube, or catheter, will be placed in another arm vein to allow us to obtain a small number of small samples of blood for analysis.
b. You will then be placed on a platform that will slide into the center or “ring” of the PET/CT scanner and a low dose CT scan from your head to your feet will be obtained. The ring will rotate around you as this occurs. This takes about 30 seconds.
c. You will then be given an injection of radioactive Copper-ETS through the intravenous catheter. We will obtain a PET scan of the heart area for 5 minutes. This will be followed by a continuous series of whole-body PET Scans that will take about 40-60 minutes. During this time, you will be required to remain quiet and, in the scanner, and in the same position as the scanner continuously moves the bed you will be laying on back and forth through the PET scanner.
d. During the Copper-ETS whole-body PET Scans 5 small blood samples (less than one teaspoon each) will be collected for analysis of the level of Copper-ETS that remains in the blood.
e. Following the PET scans, your participation is concluded and you may leave the scanner and depart.
b. You will then be placed on a platform that will slide into the center or “ring” of the PET/CT scanner and a low dose CT scan from your head to your feet will be obtained. The ring will rotate around you as this occurs. This takes about 30 seconds.
c. You will then be given an injection of radioactive Copper-ETS through the intravenous catheter. We will obtain a PET scan of the heart area for 5 minutes. This will be followed by a continuous series of whole-body PET Scans that will take about 40-60 minutes. During this time, you will be required to remain quiet and, in the scanner, and in the same position as the scanner continuously moves the bed you will be laying on back and forth through the PET scanner.
d. During the Copper-ETS whole-body PET Scans 5 small blood samples (less than one teaspoon each) will be collected for analysis of the level of Copper-ETS that remains in the blood.
e. Following the PET scans, your participation is concluded and you may leave the scanner and depart.
Incentive/compensation
The compensation will be a $100 gift card for your time and travel. Paid parking will be provided. You will only receive the compensation if the PET/CT scan is completed.
Additional information
Study title: Integrated Tools for Quantitative Whole-Body Tumor Perfusion Imaging
The study is being conducted by Mark Green, PhD, of the Department of Radiology and Imaging Sciences of the Indiana University School of Medicine. It is funded by the National Institutes of Health (NIH).
To learn more about Mark Green and his research interests, please visit this link:
https://medicine.iu.edu/faculty/4814/green-mark
Eligibility
You may be eligible for this study if you meet the following criteria:
-
Conditions:
all
-
Age: Between 20 Years - 80 Years
-
Gender: All
Inclusion Criteria
- Must be able to complete a PET scan
- Must be able to complete and understand study questionnaires and procedures in English
- Must have telephone or a reliable way in which study personal can contact them
Exclusion Criteria
- Women who are not currently pregnant, breast-feeding, or of childbearing potential and not using birth control
- Subjects who are claustrophobic an cannot tolerate imaging procedures
- Subjects who weigh > 350 lb. (upper weight limit of scanner beds)
Updated on
19 Apr 2024.
Study ID: 1902494781
Connect with a study center near you
,
You have contacted , on
Your message has been sent to the study team at ,
A copy of the message has been sent to your email
What happens next?
- You can expect the study team to contact you via email or phone in the next few days.
- Sign up as volunteer to help accelerate the development of new treatments and to get notified about similar trials.
You are contacting
Primary Contact